NAIROBI, Kenya, Jan 1 — The Pharmacy and Poisons Board (PPB) has issued a public alert warning of the circulation of falsified breast cancer medication IBRANCE® (palbociclib) in global markets, cautioning health professionals and the public to remain vigilant to prevent the products from entering Kenya.
In a statement issued Wednesday night, the Board said it received an alert from the World Health Organization (WHO) indicating that nine falsified batches of the drug — used in the treatment of certain types of breast cancer — have been identified in several countries.
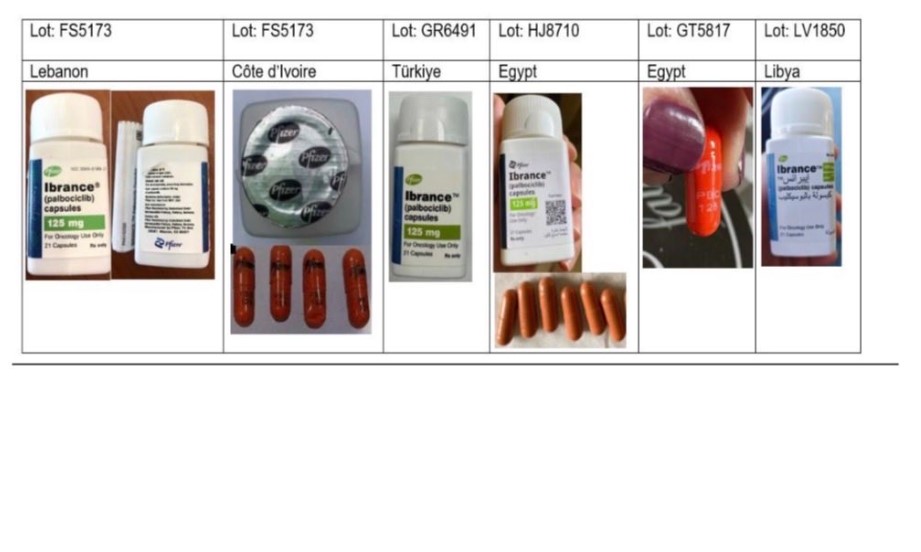
The confirmed falsified batch numbers are FS5173, GS4328, LV1850 and TS2190, while another five batches — GK2981, GR6491, GT5817, HJ8710 and HJ8715 — are considered suspicious and likely to be falsified.
According to the PPB, the counterfeit products falsely claim to have been manufactured by pharmaceutical giant Pfizer and exhibit several irregularities, including spelling errors or poor-quality printing on labels, security foil bearing the Pfizer logo printed in black ink, and capsules marked “PBC 125” or lacking markings altogether.
“The capsules have also been found to have unusual colouring, such as bright orange,” the Board said.

Laboratory analysis confirmed that the falsified products contain no active pharmaceutical ingredient, rendering them ineffective and posing a serious risk to patient safety and public health.
No reported products in Kenyan market
The Board noted that, so far, none of the falsified batches have been detected in the Kenyan market, stressing that the alert has been issued as a precautionary measure to enhance surveillance and protect the country’s medicine supply chain.
PPB urged procurement agencies, hospitals, distributors, pharmacists, pharmaceutical technologists and members of the public to report immediately any suspected cases of the falsified medicine.
“All health products and technologies should only be procured from licensed manufacturers, importers, distributors and retailers. Procuring medicines from unlicensed sources endangers patients and violates the Pharmacy and Poisons Act,” the Board warned.
The regulator said it would work with other government investigative agencies to take firm regulatory and legal action against individuals or entities found to be involved in the distribution of falsified medicines.
Members of the public can report suspected falsified or substandard medicines through the PPB’s online portal, USSD code *271#, the mPVERS mobile application, or via email and telephone channels provided by the Board.