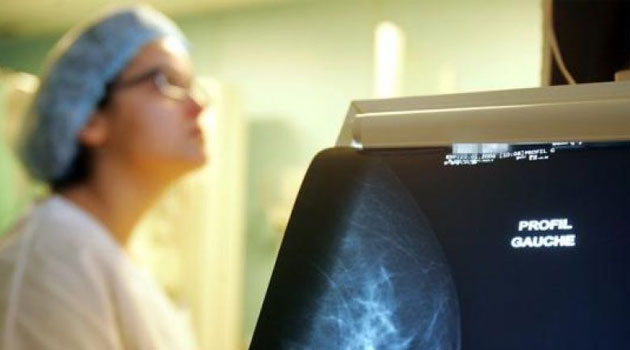
The Cobas HPV test will be available to women across the country and the region through over 40 Lancet branches in Kenya, Uganda, Rwanda and Tanzania/FILE
NAIROBI, Kenya, Aug 3 – Lancet Kenya has launched a new molecular technology that can accurately establish if one is at risk of developing cervical cancer by testing for the Human Papilloma Virus (HPV) that causes over 70 per cent of cervical cancer.
Speaking during the launch, Lancet Group Managing Director East Africa Dr Ahmed Kalebi, said the new screening technology, dubbed ‘Cobas’, tests for the presence of HPV in the cervix and cervical-vaginal canal, and gives a clear indication of a woman’s risk of developing this type of cancer, thereby enhancing efforts to effectively combat the disease in Kenya.
“Cobas specifically identifies 14 different high-risk strains of the virus that are linked directly to pre-cancerous changes, including the type 16 and type 18 strains that cause over 70pc of cervical cancer,” he said.
Kalebi said neither pap smear nor visual inspection which are current techniques used for screening for cervical cancer in Kenya, test for the presence of high risk HPV virus strains. He described ‘Cobas ‘as the new molecular technology that detects DNA of the virus
“The current tests look for abnormal changes in the cells and tissue of the cervix that may be a sign of cancer, at the point of detection it may be too late as the changes caused by the virus may be fairly advanced,” added Kalebi.
The Ministry of Health indicated that over 4,800 are diagnosed with cervical disease every year and at least 2,400 die from the disease. Kalebi however said the number can be significantly reduced with accurate screening as cervical cancer can be prevented before it develops.
Head of Management Centre sub-Saharan Africa Rajen Bhimaraj, speaking at the launch described Cobas as an outstanding example of how innovation in diagnostics is shifting the disease management paradigm to improve patient care and people’s health.
“We are committed to working with the medical community and professional organizations to put the necessary clinical practice guidelines in place, to encourage providers to incorporate this new screening strategy alternative in their patient protocols.” He said.
Bhimaraj explained that women who are found to have the virus will be classified as being at high risk of developing cervical cancer, while those who test negative will be at low risk for at least three to five years when they will need to be tested again.
“With the entry of Cobas HPV test, only a small fraction of Kenyan women 7-15pc, will still need to undergo the annual pap smear test,” he said.
He went further to say the new molecular screening technology will save many women the discomfort or embarrassment that often comes with pap smears or visual inspections where the health personnel has to insert a plastic or metal instrument (speculum) into the vaginal canal to help collect or view cervical tissues for abnormal changes associated with cervical cancer.
“With Cobas HPV test, the samples are collected by simply swabbing the inner parts of the vagina using a soft collection device called an Evalyn brush, which is specifically customised and validated for this purpose.”
Dr Rabia Mukadam, a Molecular Scientist at Lancet Kenya explained that the sample collection with the Evalyn brush can be done at home without supervision. The bar-coded brush can then be dispatched securely and /or anonymously to the lab for testing, with results delivered confidentially through email, SMS or mobile app.
“The uptake of available screening tools for cervical cancers has been low partly due to privacy concerns among many women, but the self-collection of samples for HPV testing seeks to address that barrier,” he said.
Mukadam said the technology will make the procedure easier for women because the brush that collects the sample does not even have to reach the cervix to collect adequate sample for testing but just the inner parts of the vaginal canal.
The Cobas HPV test will be available to women across the country and the region through over 40 Lancet branches in Kenya, Uganda, Rwanda and Tanzania.