Tuesday, 6th March Kenya received the first batch of AstraZeneca vaccine; a consignment of 1.02 million doses which is part of an initial allocation to Kenya, of 3.56 million doses to be distributed by the ministry of health.
As the world responds to the COVID-19 pandemic, we face the challenge of misinformation and conspiracy theories related to vaccination and with Kenya currently experiencing a peak in infection and death rate, the stakes couldn’t be higher.
Here’s what you need to know about COVID-19 vaccination in Kenya.
How does it work?
The Oxford Astrazeneca vaccine, which was manufactured by the serum institute of India is made from a weakened version of a common cold virus (known as adenovirus) from chimpanzees.
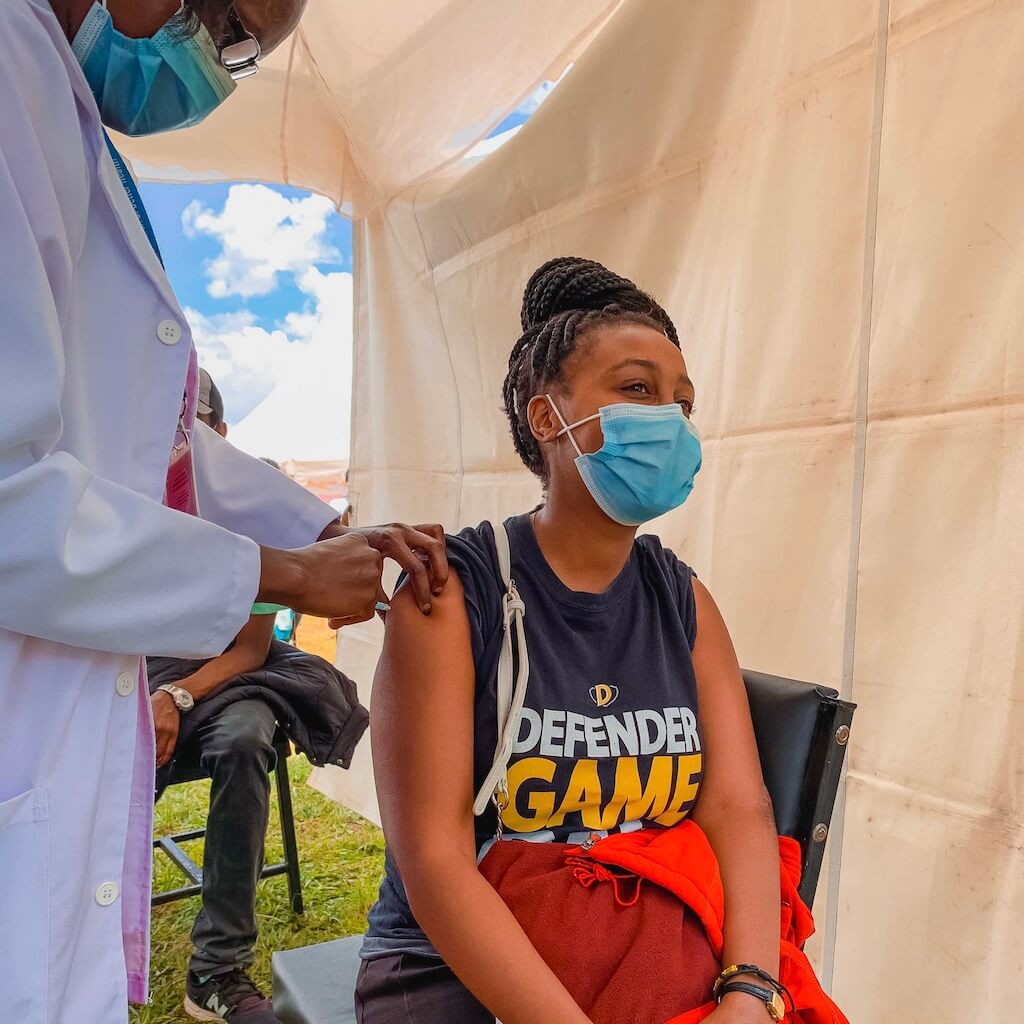
Once injected into a person’s arm the adenoviruses create an imitation of the coronavirus, teaching the body’s immune system how to fight the real virus. Read more on how the vaccine works HERE.
What is the current guidance and who should be vaccinated first?
The Ministry of Health has developed a vaccine deployment plan with healthcare providers, essential workers including security personnel and individuals above the age of 58 years being offered the vaccine first.
How many vaccines are there?
Kenya received an initial batch of 1.02 million doses earlier in March 2021, and according to an immunisation update released by Kenya’s ministry of health on 8th April 2021, a total of 487,278 persons have been vaccinated across all counties, 56.5% of those being male and 43.5% female. Full report by the Ministry of Health HERE.
How will the dose be administered?
The vaccine will be administered on the arm in two doses, to be distributed 8 weeks apart.
Where can I be vaccinated?
The ministry of health has released a list of approved COVID-19 vaccination posts across the country between March and June 2021. Comprehensive list HERE.
Who has decided the vaccine is safe to use?
AstraZeneca vaccine underwent SAGE consideration after review by the European Medicines Agency (EMA). The EMA thoroughly assessed the data on the quality, safety and efficacy of the vaccine and recommended granting a conditional marketing for persons above the age of 18, according to WHO.