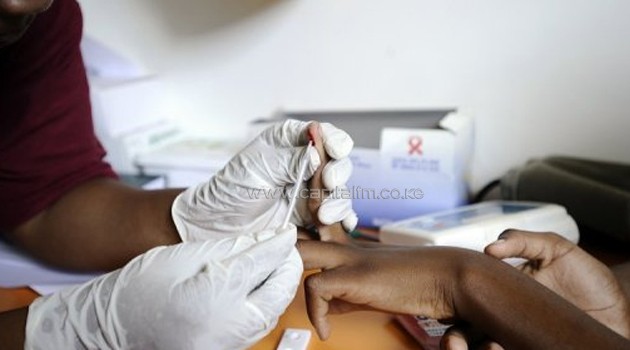
NAIROBI, Kenya, Sep 23 – The Governments of Kenya and South Africa have signed a pricing agreement which will accelerate the availability of the first affordable, generic, single-pill HIV treatment regimen containing dolutegravir (DTG).
In a statement from United Nations Programme on HIV/AIDS, the agreement targets public sector purchasers in low- and middle-income countries at around US$75 (Sh7,700) per person, per year.
The agreement is expected to accelerate treatment rollout as part of global efforts to reach all 36.7 million people living with HIV with high-quality antiretroviral therapy.
“DTG, a best-in-class integrase inhibitor, is widely used in high-income countries and is recommended by the World Health Organization (WHO) as an alternative first-line HIV regimen, as well as a preferred treatment by the U.S. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents, among many others.”
In addition to improving treatment quality and retention, widespread use of DTG is expected to lower the cost of first-line HIV treatment regimens while also reducing the need for more expensive second- and third-line regimens.
In July 2017, WHO issued guidance to countries on how to safely and rapidly transition to DTG-based antiretroviral treatment.
UNAIDS estimates that in 2016, just over half of all people living with HIV, had access to the lifesaving medicines.
“In the antiretroviral therapy guidelines launched in July 2016, the Ministry of Health made provisions for use of newer antiretroviral medicines such as dolutegravir,” said Cabinet Secretary of Health Dr. Cleopa Mailu.
“Research has shown that dolutegravir offers better tolerability, fewer adverse drug reactions, fewer drug interactions, and higher genetic barrier to resistance. It is with this in mind that, in July this year, Kenya approved its inclusion in the National ART Program,” he added.
The agreement is in partnership with the Clinton Health Access Initiative, the Bill & Melinda Gates Foundation and the United Kingdom’s Department for International Development among other partners.
“WHO welcomes this agreement which will make it possible to reach millions of people with better, more affordable and durable HIV drugs. This will save lives for the most vulnerable, bringing the world closer to the elimination of HIV. We congratulate South Africa, Kenya, Clinton Health Access Initiative and others on this landmark agreement. WHO will support countries in the safe introduction and a swift transition to this game-changing new treatment,” WHO Director-General, Dr. Tedros Adhanom stated.
According to the statement, Ministries of Health and program managers should anticipate being able to order the drugs in 2018 at around a projected average price of US$75 per patient, per year.
The ceiling price agreements apply to purchases for public sector use in all 92 countries covered under ViiV Healthcare’s dolutegravir licensing agreement, representing over 90 percent of people in LMICs currently living with HIV.